Helping you bring optimized devices and wearables to market
You'll receive the personalized attention and support you deserve when you partner with Element. From consulting on algorithm and sensor development to offering guidance on regulatory compliance, user interface, and clinical validation, we operate as part of your team, and will be by your side every step of the way. Ready to get started? Click the orange "Get more information" button below and fill out the form. A member of our team will be in touch with you.
Your partner from development to clearance
You'll reduce the risk of study failures, save time and money, and accelerate time to market when you partner with Element for pilot studies during the early stages of development. We know that time is precious, and no one wants to start over. That's why we're here to provide expert guidance and support to help you avoid costly mistakes.
Tap into our extensive in-house study participant database covering various physiologies and medical conditions for valuable insights throughout development and clinical validation.
Helping you achieve regulatory clearance
You can rely on our demonstrated history of success obtaining 510K, De Novo, and CE Mark approvals. We'll help you navigate the regulatory landscape with efficiency and precision, providing expert guidance on regulatory standards. As consultants to regulatory agencies, we offer up-to-date insights, empowering your decision making.
Plus, we'll be there to support you through pre-submission meetings with regulatory bodies, helping you obtain clearance with confidence.
Experience seamless, dedicated support
From project initiation to completion, our engineering, clinical, and regulatory experts seamlessly integrate with your development team.
In addition to delivering turnkey solutions that apply to the entire product lifecycle, we provide peace of mind - our Chief Medical Officer is present on site for all clinical validation studies, ensuring a high level of expertise and oversight. And you can rest assured your data remains under your ownership - we prioritize confidentiality and recognize how important it is for sponsors to own their data. Ready to start the conversation? Fill out our request form.
Get to know Element
Scroll down to explore a handful of the many services we offer to help you quickly achieve regulatory clearance.
Optimizing devices
To explore Element's clinical validation services, click the links below. Interested in knowing more? Keep scrolling to see a handful of our global service offerings.
Your global testing and certification partner from R&D to market
Interested in seeing what more Element has to offer? Here are a handful of our many services to help achieve regulatory clearance quickly and efficiently.

Medical Device Battery Testing
Element provides safety and certification testing for rechargeable lithium-ion and nickel metal hydride batteries used in hospital and home health applications.
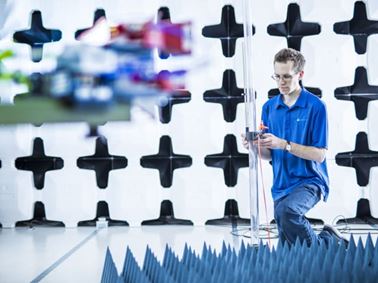
EMC Testing for Medical Devices
Find out how Element works with manufacturers of electrical medical devices to identify the testing, and certification needed for compliance to EMC safety standards and international regulations.
Medical Device Radio Testing
Our medical regulatory affairs experts can identify the appropriate Radio Testing standards and certification, helping to remove both the cost and risk from medical product verification and validation during the formal testing phase.

Wireless Coexistence Testing for Medical Devices
As more wireless medical devices enter the product development cycle, wireless coexistence testing is one of the many recommended tests that provides pertinent information for regulatory submissions.

CE Marking and Testing
CE marking is a mandatory conformity mark enabling you to enjoy free movement between all 28 Member states.
Medical Device Safety Testing
We provide medical device manufacturers with the ability to identify the appropriate safety testing standards and remove both cost and risk out of the product verification and validation process.

Additive Manufacturing
Element's Engaged Experts provide the most comprehensive level of additive manufacturing testing services in the materials testing sector.

Medical Device Regulatory Services
Our medical device regulatory experts help manufacturers of medical devices with product registrations, quality management systems, and in-country and global approvals.

