Multi-axis Testing of Peripheral Intravascular Devices
Treatment of peripheral arterial disease has long been a challenge, particularly for the superficial femoral artery (SFA). Unlike other interventions such as stenting coronary, renal, or iliac arteries, long-term patency following peripheral interventions has been difficult to achieve.
While the “gold standard” for treating SFA occlusive disease has been femoro-popliteal bypass surgery, vascular surgeons often avoid this procedure because many patients have concurrent coronary artery disease and the saphenous vein may be needed later for coronary bypass surgery. Instead, doctors first advocate a conservative approach towards alleviating walking pain due to SFA occlusive disease by focusing on exercise, pharmacotherapy, and risk factor modifications.
Unfortunately, relatively few patients achieve significant relief from symptoms with an exercise program. Pharmacotherapy is only a temporary solution to treat symptoms and offers few options to patients. Alternative treatments including statins, angiotensin-converting enzyme inhibitors, prostaglandins, and certain dietary supplements have produced inconsistent results at best. Medical devices including novel angioplasty balloons, various atherosclerosis removal devices, stents and combinations thereof have been developed for remedying diseased SFAs.
The great success of stenting in coronary arteries has led to strong interest in applying stent technology to the peripheral vasculature. However, due to the long lengths of peripheral arteries and the extreme motions that they undergo (shortening/extensions, torsion and radial compression), satisfactory long-term interventional results have been difficult to achieve with these devices. In 2006, SRI international, Stanford University, and a consortium of stent manufacturers teamed together to improve the durability of peripheral vascular implants. Their project was known as the RESIStent (Reliability Enhancement and Service Improvement for Stents) program.
Previous studies had shown very large mixed mode deformations of the SFA under the normal range of motion with significant combinations of bending, torsion, axial deformation with deformations well beyond the strains considered for coronary stent design. An important outcome of the RESIStent Program was the identification of bending, torsion, axial deformations that would simulate the in-vivo loading experienced by these intravascular devices.

The Challenge
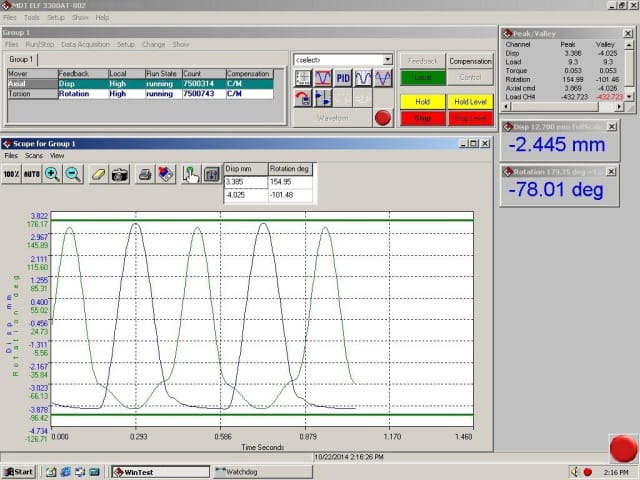
With the SFA motion specifications in hand, the challenge was to develop a test system or test fixture that could replicate these movements in vitro. One example is the ElectroForce 9500 Series Test instrument developed by Bose, which can perform a number of motions including rotation, axial extension, bending and pulsatile dilation on up to 12 stents under servo-controlled conditions. The system does not simulate crush conditions. Element owns one of these instruments and it is available and adaptable for various test needs.
Additionally, Element has developed a peripheral fixture that can be retrofitted to an existing ELF 3300 or Instron ElectroPuls 3000 Axial-Torsion system. The peripheral fixture uses the axial actuator to impart tensile/compressive strain into the device while the torsional actuator is used to provide rotation, bending and radial compression to the mock artery the device is placed within. The bending and radial compression is applied using saddles that capture and bend/squeeze the mock artery.
Range of Motion
The MDT SFA fixture is in compliance with ASTM 2942-13 and can be adapted to accommodate a wide variety of stent geometries by changing the magnitude of the applied motions and saddle geometries. Several studies have already been completed using this test fixture and it has demonstrated its ability to withstand long-term durability testing over many millions of cycles.
Conclusion
New medical device designs targeted at the peripheral vascular market (particularly SFAs) require test designs that differ dramatically from previous durability tests targeted at coronary and coronary-like devices. Already proven over numerous tests, the ElectroForce 9500 and SFA fixture developed by MDT enables MDT to perform accelerated durability testing on these new devices in vitro.
Find related Resources



