ASTM F2582: Testing Impingement of Hip Prostheses
In total hip arthroplasty (THA), cumulative advances in wear rate reduction have resulted in a decline in particle-induced osteolysis, and wear-related revisions. With this decline, hip instability and mechanical loosening have become the leading reasons for revision surgery.[1] However, dislocation and its level of unpredictability remain a significant challenge in THA.
Inferring any predisposing patient factors is difficult with any significant statistical power, even with large sample size clinical data, due to the large number of variables. Although rare well-controlled single-surgeon long-term studies exist, utilizing few changes in implant and surgical technique, the data provide benefits in terms of statistical power but lack the scope of changing key variables of interest. [2]
Hip prostheses challenges
The contributions controlled by the device manufacturer can tremendously influence instability and dislocation. Aside from surgical technique, patient disposition, and patient activity factors, variables can include head size, liner lip chamfer angle, cup insert depth, cup liner offset, or femoral stem offset. This list is illustrative, not exhaustive, to show just how many variables contribute to THA hip instability.
Element approaches the renewed focus on these challenges by leveraging its diverse and extensive history of mechanical testing experience with industry-leading biomedical simulation test equipment, as well as a range of custom designed test equipment. With this capability, our orthopedic device experts help customers understand the complex mechanisms of their hip systems for development as well as device clearance through the FDA and other regulatory bodies.
Our approach to impingement testing
Within the past 10-15 years, standardized mechanical test methods for hip impingement were developed to more closely replicate clinical failure modes. While there are many test methods for hip devices, the focus of this article is on ASTM F2582-Standard Test Method for Impingement of Acetabular Prostheses as it pertains to single-piece acetabular prostheses, modular prostheses and constrained prostheses manufactured from metallic, ceramic or polymeric materials.
The ASTM F2582 test method involves a displacement controlled test in which the acetabular liner is subject to repeated impingement from the femoral stem, while simultaneously undergoing physiologically relevant articulation and loading. From the initial reference position in which the stem is in contact with the liner, the system is articulated 0°-5° in abduction, 0°-10° in extension and -5°-5° in internal/external rotation. Figure 1 graphically illustrates the phase alignment of the motions.
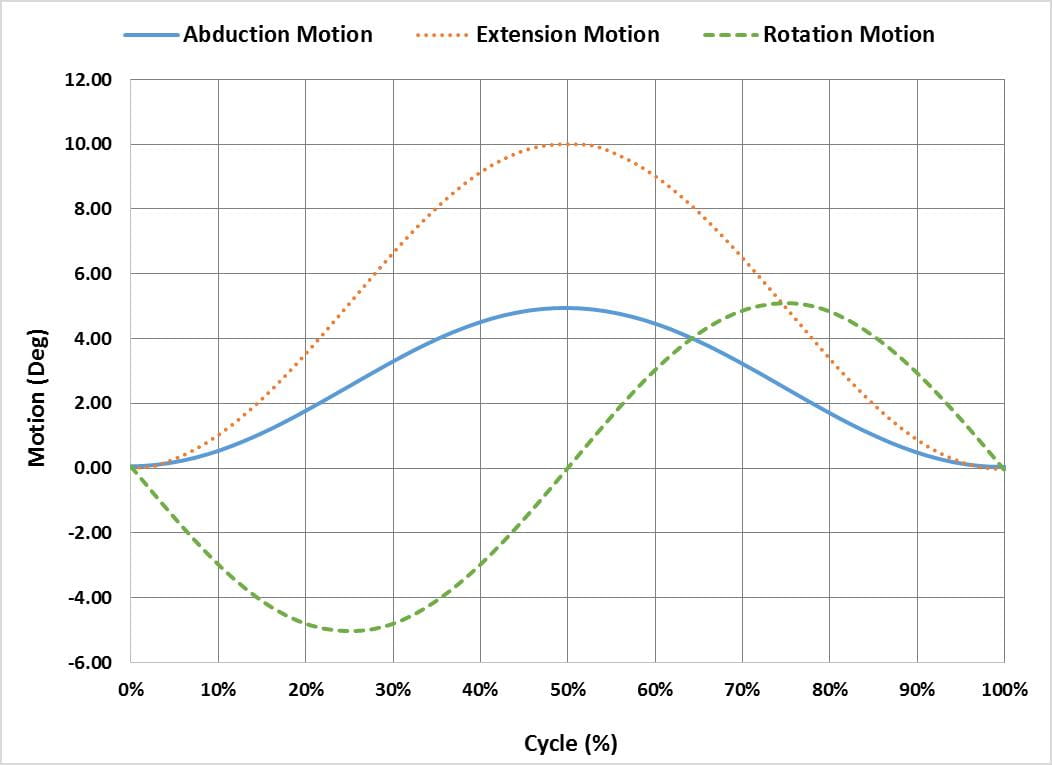
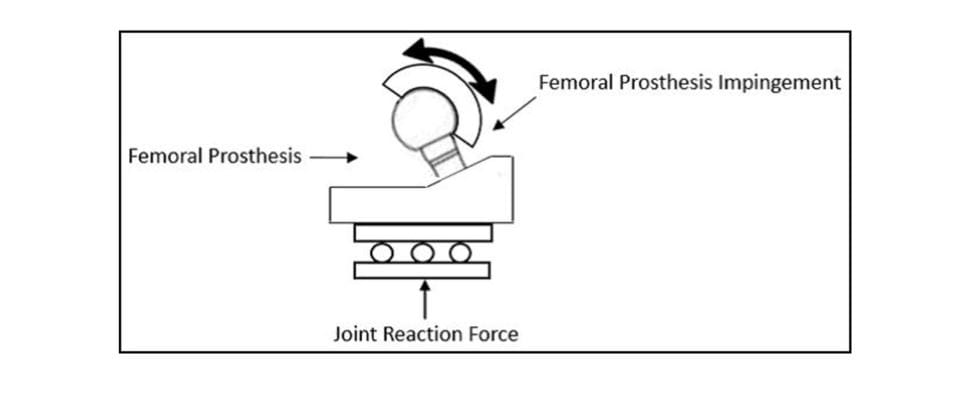
In addition to the motions, a constant 600N joint reaction force is applied. The force should be applied orthogonal to the entry plane of the liner, as shown in Figure 2 but has also successfully been applied through the stem as the liner articulates, as shown in Figure 3.

Preparing specimens for ASTM F2582 testing
The oxidation of the finished and packaged ultra-high molecular weight polyethylene (UHMWPE) liners and subsequent degradation of chemical and mechanical properties is measured in years. Preconditioning according to ASTM F2003 - Practice for Accelerated Aging of UHMWPE after Gamma Irradiation in Air aids in accounting for any degradation of the liners. The method involves immersing the liners in a pure oxygen environment at elevated pressure and temperature for two weeks.
Afterward, the surface geometry of the unworn acetabular liners is measured. A second geometry measurement is taken post-test to calculate the volume change due to impingement. Typically these measurements are taken with a structured blue light or CT scan. Solid models are created, and a scan-to-scan comparison is performed to determine volume change.
Finally, the liners are preconditioned according to ISO 14242-2, a standardized process for cleaning and weighing the liners at interval, while being soaked in a bovine calf serum solution at 37°C. The liners absorb fluid over days to weeks and are repeatedly weighed with this process until a steady rate of fluid sorption is established. The final weight measurement in this step is used as the pre-test mass for the liners, and liners are then fitted into test fixtures to begin testing.
Impingement test method
In general, the testing involves applying a constant joint reaction force of 600N and performing a series of five intervals of 200k cycles each. The femoral stem impinges on the liner rim in abduction motion while also rotating in flexion-extension and internal-external rotation. Between intervals, the impingement position is adjusted or reset to ensure contact between the stem and liner occurs throughout the entire cycle. The testing is typically performed in a solution of bovine calf serum at 37°C at a frequency of 1-2Hz, up to 3Hz. The liner is removed between intervals for periodic mass loss analysis, although this process can be omitted, as discussed further in the challenges and recommendations section.
A total of seven specimens is typically used to evaluate a hip prosthesis per ASTM F2582. Three of the specimens, termed ‘impingement specimens’, undergo the joint reaction force and abduction-adduction, flexion-extension, and internal-external rotation. Another three specimens are used as articulating controls and are tested in the same condition except they are positioned such that impingement of the femoral stem on the liner never occurs. The final specimen acts as a loaded control specimen and only experiences the joint reaction force, allowing the de-coupling of fluid absorption. Similarly, the articulating control specimens allow for the de-coupling of mass loss due to impingement from the mass loss due to wear.
Impingement specimen fixation
In most cases, the acetabular shell is carefully cemented in PMMA bone cement in a Delrin or 316SS fixture. The femoral stems are either full hip stems modified to fit in the test fixtures or machined trunnions specifically designed to fit in the test simulator while still maintaining all of the critical geometry of the final part. In either situation, the femoral stems need to be carefully machined to align the location of impingement with the desired worst-case orientation.
ASTM F2582 challenges and recommendations
There are a few parts of the standard that require knowledge of the purpose for conducting the test and careful stewardship over how the test is carried out. Additionally, it is important to know what the test can and cannot do. We will discuss a few areas that require extra attention and consideration.
One challenge is the adjustment of the abduction angle during the interval inspection periods. During this step, the stem is rotated in abduction motion until it impinges on the liner, and this location becomes the new starting point for abduction motion. Adjusting the device on the test frame presents challenges due to the small test components and curved surface features, as well as the relatively large range of abduction motion that can impede access to the components. Careful visual inspection and adjustment of the device are crucial. One possibility to make this adjustment more objective would be to rotate the stem in abduction motion until a pre-defined moment is achieved. The rotation could be performed with a 6-axis load cell so that the precise starting point could be located at each interval.
Another area of concern is the relatively small amount of mass loss achieved during the test. This can be on the order of only a few milligrams per million cycles. Mass loss is further obfuscated when trying to correct for fluid uptake, or when separating mass loss due to wear from the mass loss due to impingement. With a sample size of only three impingement specimens and three articulating control specimens, the variation between these two groups is sometimes undetectable.
Furthermore, if the liners are removed from their shell every 200k cycles for mass loss analysis, any small changes that may be present could be obscured. Many times the intermittent removal and mass analysis of the liners is omitted in favor of a simple pre- and post-test measurement. Another reason for omitting liner removal is that a trend of mass loss due to impingement is not as important as the total mass loss upon test completion. Even so, mass loss from wear is rigorously tested separately per ISO 14242 or similar standards.
If interval mass analysis is to be performed, one successful technique for the removal of the liners from shells is the utilization of liquid nitrogen. Due to the dissimilar thermal expansion of the polyethylene liner and the titanium shell, exposing the acetabular components to liquid nitrogen allows for easy removal with no damage to the liner or the locking mechanism. This method works so well that the liner can be removed from the shell with liquid nitrogen without the use of any other tools to pry or force the liner.
Finally, the failure criteria defined by ASTM F2582 lack measurable detail to determine liner performance objectively. Among other things, the standard defines failure as “gross deformation of any prosthetic component.” The definition is ambiguous and difficult to apply consistently because every system exhibits permanent liner deformation at the site of impingement. Also, in some systems, there is metal-on-metal contact, which can generate a lot of harsh particulates. While this is not addressed in the standard, it is easy to see how damaging this would be in the field. Additionally, the failure criteria do not state anything in regards to the mass loss analysis or volume difference measurements and how to interpret these results. These measurements are simple to report, but the interpretation of the significance of these results would need further analysis.
Conclusion
As impingement of the stem and liner is imminent in-vivo, it is important to characterize and understand how the hip prosthesis will perform in this condition. The ASTM F2582 method does not purport to recreate in-vivo conditions accurately. It does, however, provide a standardized means for assessing the functional performance of an acetabular prosthesis under laboratory conditions. Even without clear failure definitions, the test method is useful for systematically study varying prosthesis design considerations. Depending on various goals, investigators may choose to deviate from the test method to look at other failure modes or test parameters in more detail.
Reference:
[1] Bozic, Kevin J., et al. "The epidemiology of revision total hip arthroplasty in the United States." JBJS 91.1 (2009): 128-133.
[2] Brown, Thomas D et al. “Impingement and dislocation in total hip arthroplasty: mechanisms and consequences.” The Iowa orthopedic journal vol. 34 (2014): 1-15.
ASTM F2582-14 Standard Test Method for Impingement of Acetabular Prostheses
The Element advantage
Our Engaged Experts excel at orthopedic device testing and have worked with many challenging device designs and test setups over the last several decades. We strive to meet all your medical device testing needs in the most expedient, efficient and responsive way. Contact us to discuss how we can help with your test project.
Find related Resources
Get white papers, updates and event invites
Subscribe to content updates
Related Services

Orthopedic Implant Testing
As a global leader in orthopedic implant testing, Element has years of experience in evaluating hip replacements, knee prostheses, spinal devices and many other implants.

Preventing Fretting Corrosion of Orthopedic Devices
The human body is a corrosive environment that can compromise the performance of orthopedic devices. The test methods outlined in this article aid in preventing fretting corrosion in your device designs.

Test Protocol for Medical Devices
A protocol and plan will mitigate your risk, prevent confusion, set clear expectations, and preserve the necessary information for future reference and use.
Biomechanical Testing of Cadaveric Specimen
Cadaveric biomechanical testing is one of the most beneficial forms of mechanical testing in the medical industry. Our medical device testing experts explain the advantages and challenges of testing with cadaveric specimens.
