Why Are Uncertainty Factors and Relative Response Factor Databases Important When Selecting an E&L Provider?

Kristin is a Senior Scientist at Element in our Bend, OR location. She has three years of experience in extractable and leachables testing and six years of experience and specializations in the structural elucidation of small molecule unknowns.
When selecting an extractable and leachable (E&L) service provider, it is important to select a contract lab that has the experience and data to support an appropriate Analytical Evaluation Threshold (AET) and analytical Uncertainty Factor (UF). Relative Response Factor (RRF) databases play a two-part role in E&L studies. First, RRF databases are utilized to adjust the estimated concentrations of the extractables or leachables within a profile based on known detector response differences of authentic and surrogate standards. Secondly, RRF databases are utilized to determine the UF of a particular analytical method, which directly affects the AET and indirectly determines the complexity of an E&L profile. Namely, a larger EF creates a lower AET, thereby increasing the number of extractables or leachables included in the profile. Therefore, the UF plays an important role in the number of compounds that ultimately have to undergo a toxicological assessment, which can impact patient safety risk, timelines, and cost. Element has carefully and thoughtfully invested in establishing relevant RRF databases which can be utilized to both calculate the UF and adjust the quantitation of reported compounds with authentic standards or selection of an appropriate surrogate compounds. This rich set of data helps ensure patient safety, reduce overall cost, and reduce time to market.
Introduction
Pharmaceutical manufacturing equipment1, drug product container closure systems2,3 (CCS), and medical devices4 must undergo testing for extractables and (E&L) to ensure patient safety. Extractables are substances extracted from manufacturing equipment, CCSs, and medical devices under laboratory conditions (e.g., appropriate solvents, techniques, and conditions), whereas leachables are substances that are extracted under conditions of intended use. Often leachables are a subset of extractables. For the purpose of this white paper, the terms extractables and leachables are utilized interchangeably. Leachables have the potential to adversely affect the safety, stability, efficacy, or overall quality of the packaged drug product or medical device. Due to the potential safety risks to patients and/or recipients, governing agencies, including the Food and Drug Administration and European Medicines Agency, require leachables to undergo toxicological assessment. A toxicological assessment can be time consuming and costly if a leachable profile contains a high number of compounds or is sufficiently complex. To determine which leachables are at a concentration high enough to adversely affect patients/recipients, the industry evaluates lachables using various thresholds.
Calculation of Analytical Evaluation Thresholds (AETs)
The analytical evaluation threshold (AET) is considered the level above which all leachables should be identified. In short, the AET converts a dose-based threshold into a concentration that can be used by analytical techniques to produce the leachable profile. Two types of dose-based thresholds can be utilized in E&L studies: the safety concern threshold (SCT) and the threshold of toxicological concern (TTC) The SCT establishes the threshold below which leachables would present minimal carcinogenic and non-carcinogenic safety concerns to the patient (unless the compounded is a 'special case compound'). Alternatively, the TTC establishes an acceptable intake for any unstudied chemical that poses a negligible risk of carcinogenicity or other toxic effects. The PQRI recommends an SCT for Orally Inhaled and Nasal Drug products5 and Parental Drug Products6 of 0.15 μg/day and 1.5 μg/day, respectively. Whereas the ISO/TC 21726:20197 establishes a TTC range of 1.5 to 120 μg/day, depending on the duration of the treatment.
The AET is calculated as follows (Eq. (1)):

What is Analytical Uncertainty?
Once an initial AET is derived, the AET must then be adjusted to account for analytical uncertainty. Analytical uncertainty can originate from several sources, such as:
- Uncertainty in the proposed structure and elemental composition of unknown leachables
- Uncertainty in the response of an unknown leachable with respect to the detection and quantitation of a particular analytical technique
- Sample matrix effects and interference
- Variation in sample preparation (e.g., technique, concentration factor, percent recovery)
- Quantification strategy employed
In other words, the practical problem with utilizing an AET, is that, during untargeted screening, it is impossible to optimize all analytical variables (e.g., mode of detection, reduction of sample matrix effects, optimization of sample preparation and concentration, method optimization for chromatography improvements) to mitigate analytical uncertainty like would be done for targeted analyses. For example, take a 1-ppm mixture of six E&L relevant compounds which are all detectable by ESI-LC-UV/MS, which has three possible modes of detection, UV, ESI positive, and ESI negative. Each of the six compounds can be detected by one or more modes of detection and each compound may have a different signal strength in each mode of detection. For example, Bisphenol A (BPA) (orange box in Figure 1) is detected in UV and ESI negative but produces no ESI positive signal. If you compare the strength of the signal of BPA to mono-2-ethylhexyl phthalate (MEHP) (purple box in Figure 1), BPA produces a larger signal in UV – 230 nm but a smaller signal ESI negative. The ratio of peak response (e.g., peak area) to concentration of the compound causing the peak is known as the response factor (RF); however, as shown each compound can produce different RFs based on the mode of detection or analytical technique. RFs can also vary depending on other study design choices. For example, RFs in organic versus aqueous matrices (i.e., extraction solvents) can be different due to solubility differences, ionization efficiency differences, and other factors.

A response factor can only be calculated when the concentration of the analyte is known. However, during untargeted analyses, like E&L screening, the concentration of analytes is unknown. Therefore, during untargeted analyses a relative response factor (RRF) is employed for estimating concentrations. RRFs utilize an internal or external standard at a known concentration as a “ruler” by which the AET equivalent response can be established or by which the concentration of unknowns can be estimated, see Eq. (2).
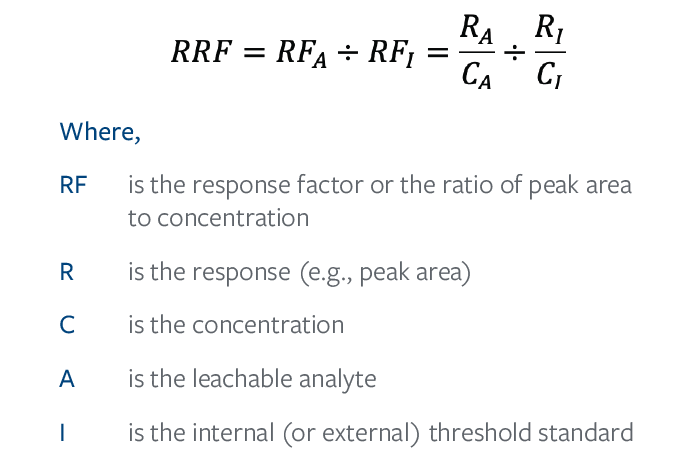
The RRF for the chosen threshold standard is therefore 1. Analytes who respond more strongly than the chosen standard have an RRF>1, whereas analytes who respond more poorly than the chosen standard have an RRF<1.
The choice of standard is then critically important to reduce the number of compounds whose concentrations are either over- or under- estimated during E&L screening. If an analyte has an RRF>1 the concentration will be overestimated and if an analyte has an RRF<1 the concentration will be underestimated. Take Figure 1 for example, if MEHP (purple box) is the standard by which the concentration of BPA (orange box) is estimated, then in UV the concentration of BPA would be overestimated while in ESI negative the concentration of BPA would be underestimated. Overestimating the concentration of leachables can cause unnecessary toxicological concern and regulatory scrutiny, as, generally speaking, the higher the concentration the greater the toxicological concern. On the other hand, underestimating concentrations can pose a risk to patient safety, as the toxicological concern of a leachable may be understated.
How is Analytical Uncertainty Accounted For?
The above examples are relatively rudimentary examples of analytical uncertainty, but, nonetheless, demonstrate the need to account for uncertainty within an AET. To account for the analytical uncertainty, the AET is adjusted by dividing the AET (initial) by an uncertainty factor (UF) as shown in Eq. (3).

Currently there are two different approaches depending on the test article application. For drug substance or drug product related applications the generally accepted approach is a UF of 2 (50% of the AET).4,5 For medical device applications an UF of 4 and 10 GCMS and LCMS techniques, respectively, is generally accepted unless a lower UF can be scientifically justified. In other words, the AET is ultimately being adjusted to account for the known variation in the RRFs of a population of relevant analytes when compared to an internal or external standard. Using Figure 1, if only ESI negative was considered for screening compounds and MEHP was selected as the standard (e.g., only compounds with a response equal to or greater than MEHP would be included in the leachable profile), then BPA would not be included in the leachable profile, despite BPA being present at 1-ppm. Because each compounds have different RFs, the AETf must be adjusted to ensure all compounds at the AETi are included in the leachable profile. It is worth noting that because RRFs are utilized for untargeted E&L screening the chosen internal or external standard plays a critical role in the calculation of UF.
Hypothetical Example of Utilizing Different Uncertainty Factors
To demonstrate the power of an UF, consider a medical device E&L study where: an TTC of 120 µg/day was established, 1 component would be utilized per day according to intended use, and 10 components were extracted in 100 mL of solvent.
The AET utilizing the recommended UF for LC would be: 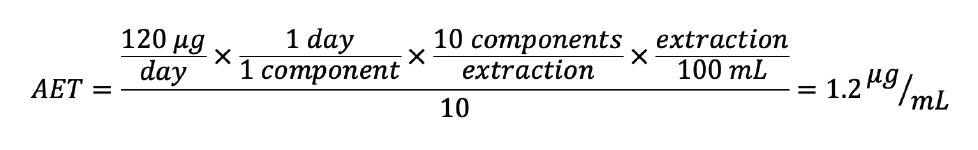
However, if an UF of 5 could be scientifically justified, then the AET would be doubled or 2.4 µg/mL. Figures 1a-c demonstrate the approximate integration threshold of the two AETs in the three possible modes of detection. In this example, utilizing an UF of 10 a total of 26 compounds would have to be processed and reported, whereas only 6 compounds would have to be reported utilizing an UF of 5.
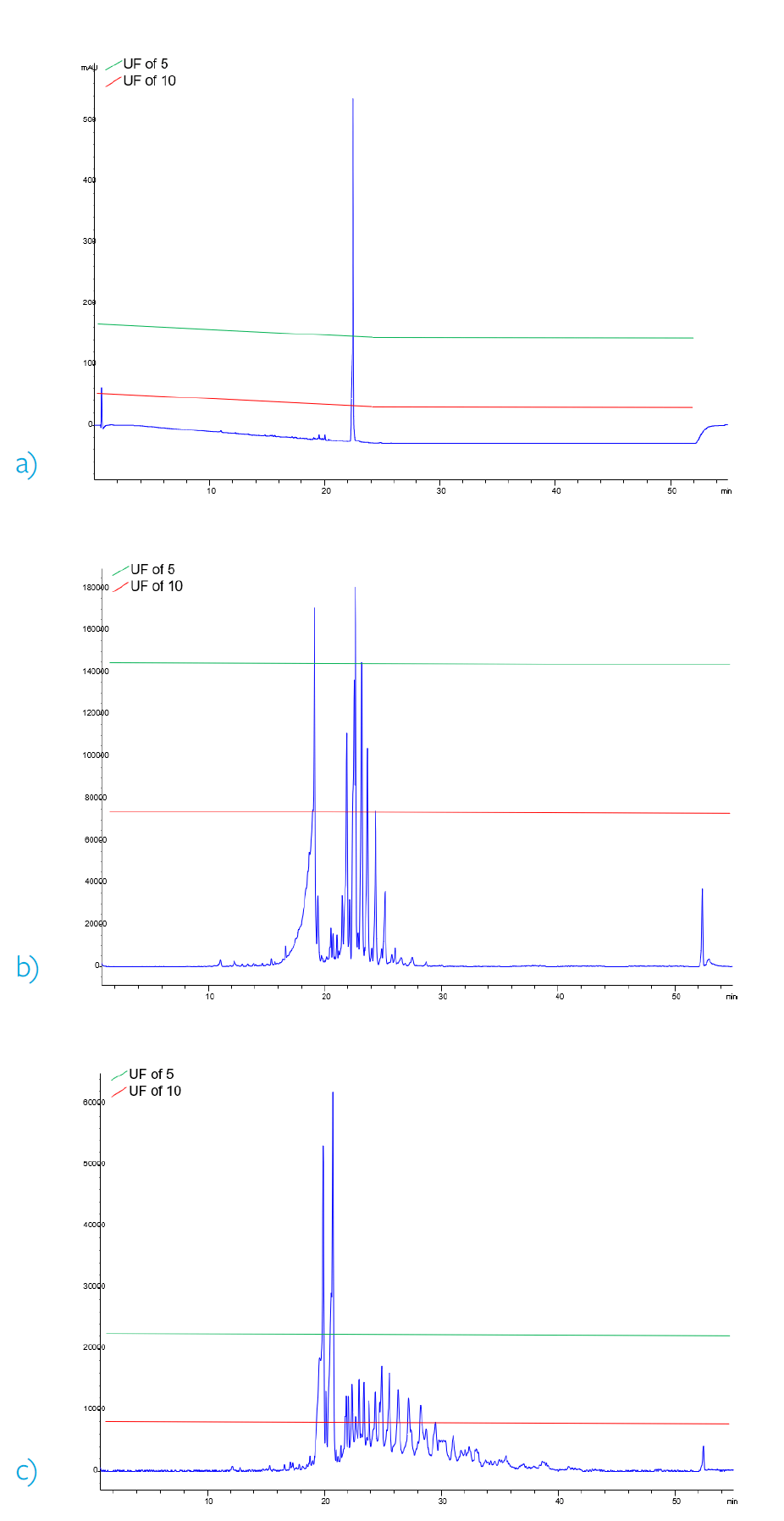
Figure 2. Comparison of approximate integration thresholds corresponding to different UF. a) 230 nm UV chromatogram b) ESI positive base peak chromatogram c) ESI negative base peak chromatogram.
Calculation of and Scientific Justification for Lower Uncertainty Factors
The above example demonstrates the importance of UFs in determining how complex a leachable profile is, but how are lower UFs scientifically justified?
Per ISO 10993-182, the UF can be calculated as:
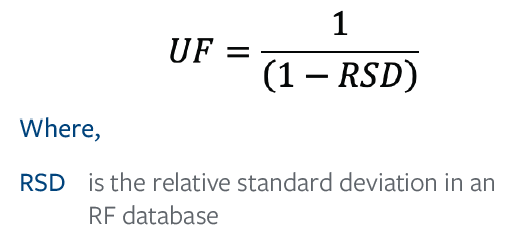
A response factor database is a compilation of RFs or, in the case of untargeted screening, RRFs. The population of compounds within a database must be appropriate for the analytical technique and should account for sample preparation and concentration. The databases developed by our E&L Center of Excellence, Element, work to account for the largest type of analytical uncertainty within each technique, including sample preparation and percent recovery.
Note: The PQRI utilizes slightly different definitions for UF and AETf, however, the overall mathematical outcome is the same. 4,5 For simplification purposes, only the ISO 10993-18 definition has been shown in detail.
LC-UV/MS
As stated above, ESI-LC-UV/MS has three modes of detection UV, MS positive, and MS negative, and analytes can be detected in one or more of these modes. The presence or absence of a signal and signal strength in comparison to the standard in a mode of detection had to be accounted for within the database. Therefore, the LC-UV/MS UF database specifically considers the mode of detection best suited for each analyte within the database.
GC-FID/MS
Samples for GC-FID/MS often go through sample preparation and concentration to convert the sample extraction solvent to a solvent suitable for injection onto the instrument. Because sample preparation and concentration are often required, the analyte population within the UF database created by Element has also undergone sample preparation. Additionally, Element currently utilizes 3 internal standards for GC-FID/MS, the RRF compared to each internal standard is considered within the UF calculation.
HDSP-GC-FID/MS
The determination of HDSP-GC-FID/MS UF poses some obstacles due to the nature of the analytical technique. Detection of analytes via HDSP is complex, as each analyte has a unique partitioning coefficient causing the detection range (both detection and quantification) to vary significantly. The partitioning co-efficient is dependent on the analyte volatility, solubility, and other chemical properties. In other words, the sensitivity of each analyte on the instrument is so variable that calculating the UF of the analytical technique must account for this variation. The HDSP-GC-FID/MS UF database created by Element not only considers the analyte sensitivity but also the impact of choosing a surrogate compound for quantification when an authentic standard is not available.
Correcting for Under- and Over- Estimations of Leachables
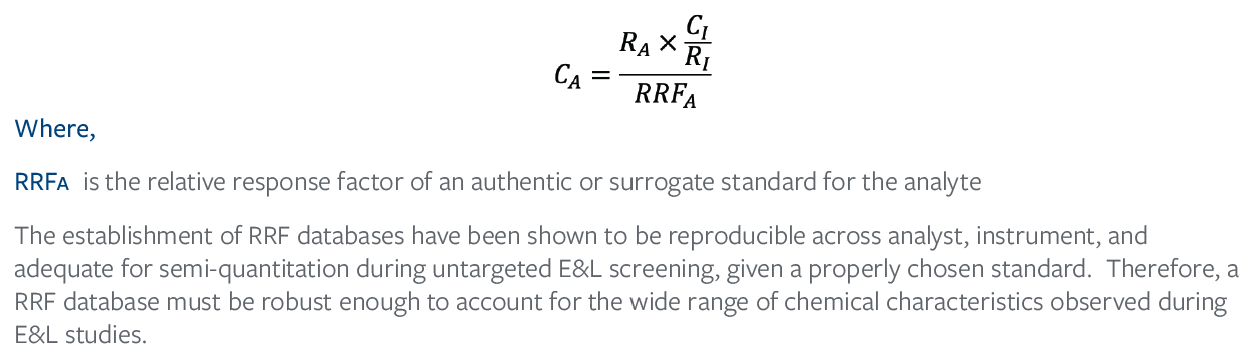
Once an AET has been properly adjusted for analytical uncertainty and a leachable profile has been established with as many identities as possible, the RF differences between the standard and leachable/analyte must still be accounted for. As discussed above, concentrations will be overestimated for analytes with an RRF>1 because the signal of the analyte is stronger than that of the standard. The opposite is also true, concentrations are underestimated for analytes with an RRF<1 because the signal of the analyte is smaller than that of the standard. However, if the RRF of an authentic standard or a surrogate standard is known, the estimated concentration can be adjusted to account for those RF differences. Therefore, rearranging Eq. (2) yields Eq. (5).
Conclusion
When selecting an E&L provider, one important aspect is whether the provider has an established, robust, and comprehensive Relative Response Factor (RRF) database. RRF databases are crucial to both the justification of lower uncertainty factors and for the concentration adjustments for more accurate quantitation of known analytes. An RRF database must account for the unique aspects that contribute to uncertainty within each analytical technique. For example, sample preparation and concentration contribute to GC-FID/MS analytical uncertainty, whereas sample preparation is often not required for LC-UV/MS and therefore does not need to be emphasized. Establishing RRF databases which reflect the analytical uncertainty of a whole technique impacts how much the AET must be adjusted thereby reducing the complexity of a leachable profile and ultimately impacting patient safety, timelines, and cost. Element has been able to establish scientifically justifiable UFs for LC-UV/MS, GC-FID/MS, and HDSP-GC-FID/MS and all UFs are lower than the medical device recommendations. The established UFs are being actively utilized in E&L studies to adjust AETs and the comprehensive RRF databases have been proven by years of utilization in E&L studies to improve estimated concentrations of known leachables. Reach out today for more information on how our Relative Response Factor databases can improve your E&L study!
Notations
¹ United States Pharmacopeia and National Formulary <665> “Plastic Components and Systems used to Manufacture Pharmaceutical Drug Products and Biopharmaceutical Drug Substances
and Products” Official as of 01Dec2020.
² United States Pharmacopeia and National Formulary <1663> “Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery Systems”. Official as of 01Dec2020.
³ United States Pharmacopeia and National Formulary <1664> “Assessment of Drug Product Leachables Associated with Pharmaceutical Packaging/Delivery Systems”. Official as of
01Dec2020.
⁴ ISO 10993-18:2020, “Biological evaluation of medical devices – Part 18: Chemical characterization of medical device materials within a risk management process.”
⁵ Product Quality Research Institute (PQRI), Leachables and Extractables Working Group. Safety Thresholds and Best Practices for Extractables and Leachables in Orally Inhaled and Nasal
Drug Products; Product Quality Research Institute: Arlington, VA, 2006.
⁶ Product Quality Research Institute (PQRI), Leachables and Extractables Working Group. Safety Thresholds and Best Demonstrated Practices for Extractables and leachables Parenteral
Drug Products (Intravenous, Subcutaneous, and Intramuscular); Product Quality Research Institute: Arlington, VA, 2021.
⁷ ISO/TS 21726:2019, “Biological evaluation of medical devices: Application of the threshold of toxicological concern (TTC) for assessing biocompatibility of medical device constituents”.
⁸ Zdravkovic, et. al. Establishment of a reference standard database for use in the qualitative and semi-quantitative analysis of pharmaceutical contact materials within an extractables survey
by GC–MS, Journal of Pharmaceutical and Biomedical Analysis, Volume 151,2018, Pages 49-60. DOI: 10.1016/j.jpba.2017.12.054.
Find related Resources
Related Resources
More from Element

Excipient Raw Materials and Container Testing
Find out how we make certain that quality and safety of your excipient raw materials and containers are maintained.

ISO 18562 Testing: Biocompatibility Analysis
Element provides ISO 18562 biocompatibility analysis for respiratory and ventilation devices to support global device approval.

CMC Product Development Services
Find out how our CMC development services support clinical trials and accelerate the overall drug development process for our customers

Extractables and Leachables Studies
Our extractables and leachables studies offer tailored solutions that ensure patient safety and compliance with industry standards.